|
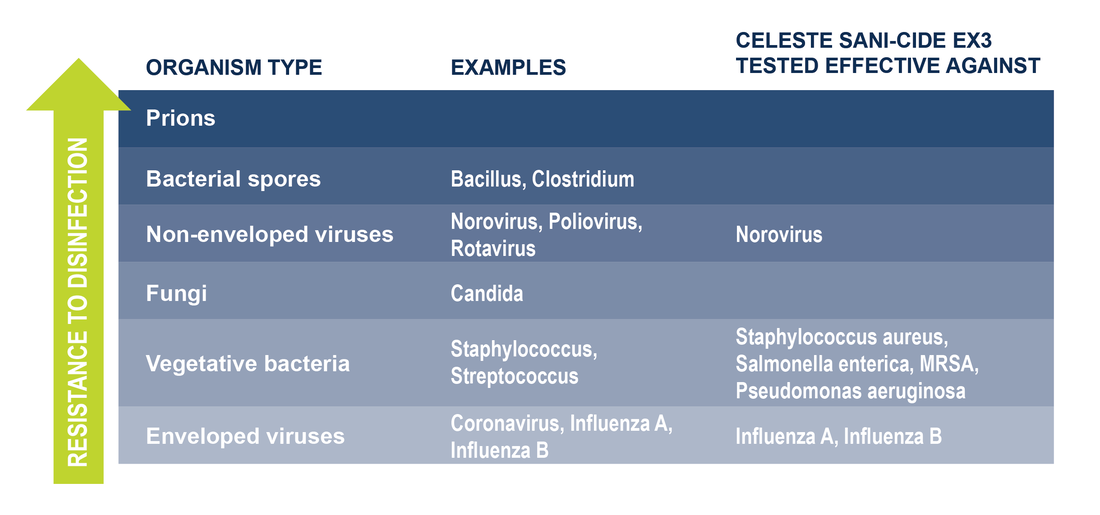
Originally Published 3/18/2020 Not only has it taken over our conversations and media feeds, Coronavirus has become a global pandemic. More than anything, the virus has made everyone focus on the importance of reducing the spread of germs. Airlines are no exception. They have been working diligently to disinfect aircraft surfaces often to keep guests safe and comfortable on their flights. Here at Celeste, we continue to produce aircraft-approved surface disinfectants, hand soaps, and hand sanitizers during this time of increased demand.  Celeste's Sani-Cide EX3 is a broad spectrum hard surface disinfectant/cleaner trusted throughout the aviation industry and registered with the US Environmental Protection Agency (EPA). To date, Sani-Cide EX3 has been tested to be effective against Feline Calicivirus (a surrogate for Norovirus), Pseudomonas aeruginosa, Methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus aureus, Salmonella enterica, Influenza A, and Influenza B. While Sani-Cide EX3 has not yet been specifically tested to kill the Coronavirus, it has been tested to be effective against similar enveloped viruses such as Influenza A and Influenza B, as well as more resistant non-enveloped viruses such as Norovirus. We are currently having Sani-Cide EX3 tested for label claims against Human Coronavirus and we will be submitting Sani-Cide EX3 for testing against SARS-CoV-2 when testing is available. Sani-Cide EX3 has been included on EPA’s List N: Disinfectants for Use Against SARS-CoV-2. List N includes products that meet EPA’s criteria for use against SARS-CoV-2, the novel Coronavirus that causes the disease COVID-19. Sani-Cide EX3 has demonstrated effectiveness against viruses similar to SARS-CoV-2 on hard, non-porous surfaces. Therefore, Sani-Cide EX3 can be used against SARS-CoV-2 when used in accordance with the directions for use against Feline calicivirus, Strain F-9, ATCC VR-782 (Surrogate for Norovirus) on hard non-porous surfaces. Refer to the CDC website at www.cdc.gov for additional information.  Of course by now, we have all heard that washing your hands regularly is one of the best ways to prevent the spread of illness as recommended by US Centers for Disease Control and Prevention (CDC), European Centre for Disease Prevention and Control (ECDC), and World Health Organization (WHO). Celeste offers several hand washing products trusted in the aviation industry. Our Flight Luxe® Hand Soap, available in liquid or foaming formulas, is an above-the-counter soap system making replacing soap an easy task. Simply remove the old bottle and replace with a brand new one! What about antibacterial soaps? According to the US Food and Drug Administration (FDA) and CDC, there is no evidence to show that consumer antibacterial soaps are any better at preventing illness than washing with plain soap and water.  If soap and water are not available, and hands are not visibly dirty, it is recommended to use hand sanitizer with at least 60% alcohol, if available, by applying the product to the palm of one hand and rubbing the product over all surfaces of your hands and fingers until dry. Our Sani Luxe® Hand Sanitizer, bottled in the same convenient system as our hand soap, is available in 3 formulas (availability may vary by country):
For more information on Celeste products, speak with your Account Manager or Sales Coordinator. For more information on Coronavirus: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 https://www.cdc.gov/coronavirus/2019-ncov/index.html https://www.ecdc.europa.eu/en/novel-coronavirus-china
16 Comments
Maintaining Safety and Standards Through Good Manufacturing Practice and Good Laboratory Practice6/29/2018 Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP) go hand in hand to maintain high safety and quality standards. GMP ensures the safety of the end user by controlling the manufacturing of the product, whereas GLP ensures the quality of the product by ensuring all activities are performed in a consistent manner. Both GMP and GLP are regulated by the US Food and Drug Administration (FDA) and affect our production of antibacterial hand soaps and hand sanitizers.
Good Labortatory Practice is a quality system of management controls that exists to guarantee the consistency, reliability, and quality of chemical tests. Following GLP ensures that when we test our products for efficacy and quality, the results are reliable and can be reproduced. This allows us to consistently check our products for any type of contamination and protect the end user from harm. Good Manufacturing Practice (GMP) guidelines exist to protect the end user of a product from harm. These guidelines provide direction for manufacturing, testing, and quality assurance to ensure that a final product is safe for human use or consumption. In the United States, the Food and Drug Administration (FDA) requires companies to follow GMP when manufacturing certain products. In our case, it affects our manufacturing of antibacterial hand soap and hand sanitizer.
|
Archives
May 2020
Categories
All
BLOG SUBSCRIPTION
|
800.447.5775
[email protected]



 RSS Feed
RSS Feed

